Next: Detonations with One-Step Chemistry Up: Multi-component Euler Equations Previous: Multi-component Euler Equations
Reaction Terms of Detailed Chemistry
The reaction terms for detailed chemcial reaction are determined by a reaction mechanism of M chemcial reactions, i.e.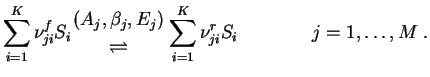
![$\displaystyle \dot \omega_i = \sum_{j=1}^M (\nu_{ji}^r-\nu_{ji}^f)\biggl[ k_j^f...
...{n=1}^K\Bigl({\rho_n\over W_n}
\Bigr)^{\nu_{jn}^r} \bigg] \quad i=1,\dots,K\;, $](img13.gif)
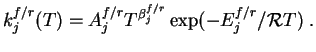
Table of Contents Home Contact